
/Atom-58e4f7173df78c51626061cc.jpg)
/GettyImages-517345224-e456b6c739d64be89a519da75bb63e06.jpg)
#ATOMIC NUMBER FULL#
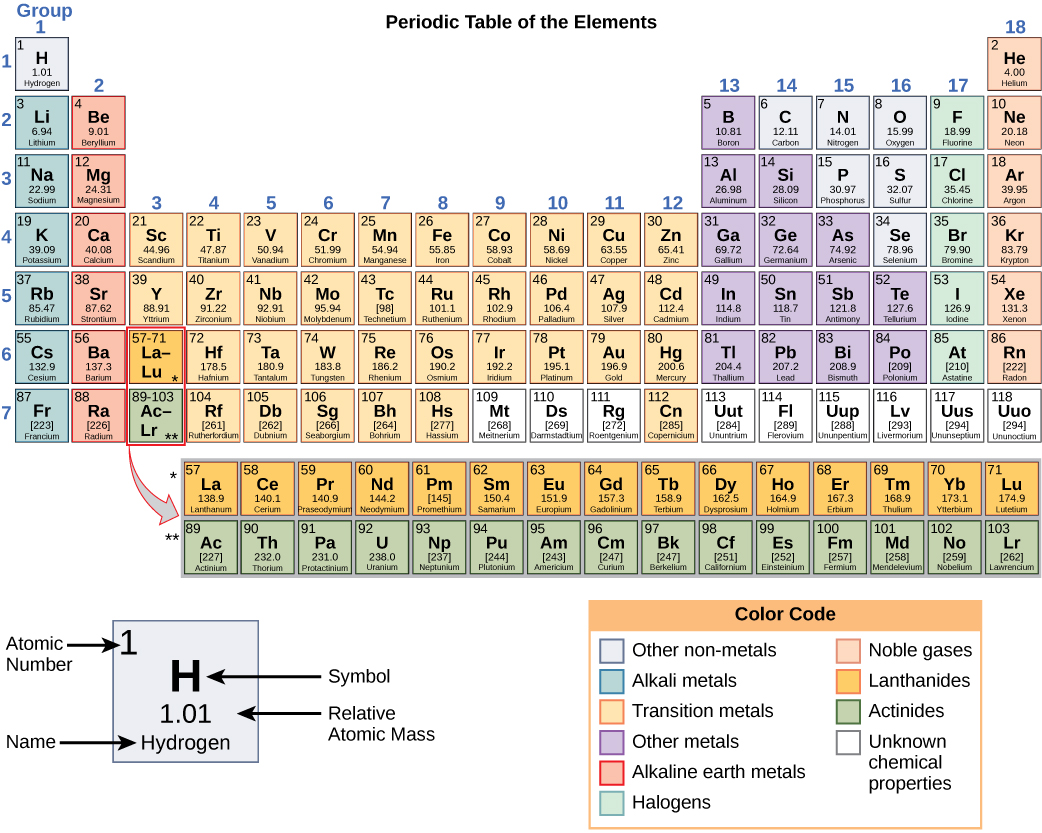
The original paper should be consulted for full details of the variation in atomic weight and the half life of the radioisotopes quoted below.Ī number in parentheses indicates the uncertainty in the last digit of the atomic weight. For radioactive elements the isotope with the longest half-life is quoted in parenthesis. In the other lists the values quoted are those suggested for material where the origin of the sample is unknown. The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-weight values in natural terrestrial materials. Previous values may be consulted from the 1993 table, the 1995 table, the 1997 table, the 1999 table, the 2001 table, the 2005 table, the 2007 table, the 2009 table, the 2011 table, the 2013 table, the 2015 table or the 2019 table. World Wide Web version of atomic weight data originally prepared by G. These tables are based on the 2021 table with changes from the 2019 table for the values of Ar, Hf, Ir, Pb and Yb and changes to the uncertainty for Al, Au, Co, F, Ho, Mn, Nb, Pa, Pr, Rh, Sc, Tb, Tm, and Y. Atomic mass is the total mass of an atom’s protons, neutrons, and electrons.2021 Atomic Weights IUPAC Commission on Isotopic Abundances and Atomic Weights. Difference Between Atomic Number and Atomic MassĪtomic mass is different from atomic number.
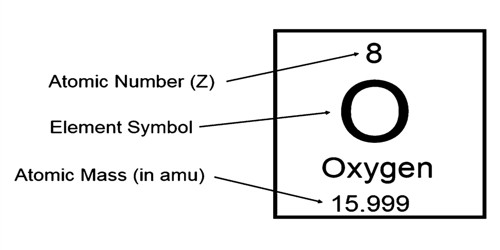
When the number of electrons doesn’t match the number of protons, an “atom” is actually an ion. That’s why we can usually touch matter made of atoms without getting electrical shocks. The balancing or “canceling” of charge makes an atom electrically neutral. The two, electrons and protons, balance each other’s electrical charge. Image source: Ordinarily, an atom has the same number of electrons as it has protons. Periodic Table of the Elements featuring the atomic number of each element. In the copy of the table which appears in this article, the atomic number is at the center of each cell. The Periodic Table of the Elements lists the elements in the sequence of their atomic number. Atomic Number in the Periodic Table of the Elements In other words, for the most part, the differences between the elements, carbon and oxygen, stem from the difference in the number of their protons, their atomic number. The atomic number determines the chemical element. This is true for any oxygen atom in the universe. It is always fixed for a given element, for example, the atomic number of an oxygen atom is 16. The mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. An atom of oxygen has eight protons and has Atomic Number 8. The atomic number is the number of protons present in the nucleus of an atom. For example, an atom of carbon has six protons and has Atomic Number 6. The atomic number of an atom is the number of protons in its nucleus.


 0 kommentar(er)
0 kommentar(er)
